Surfactant controlled magnesium oxide synthesis for base catalysis†
Abstract
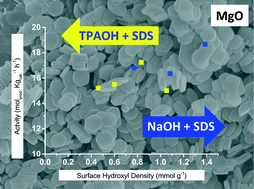
Magnesium oxide catalysts were used to investigate the influence of novel preparative techniques for surface site control on activity. Firstly, magnesium hydroxide was prepared under hydrothermal conditions with sodium dodecyl sulphate and either sodium hydroxide or a quaternary ammonium ion as morphological and surface controlling agents. Particles with a hexagonal, plate-like morphology were produced with varying dimensions relative to the concentrations of surfactant to ammonium ion. These materials were dehydrated at 420 °C for 2 hours and then characterised by nitrogen adsorption, X-ray diffraction, electron microscopy and the surface hydroxyl density was measured via1H MAS NMR. Analysis of electron microscopy images of the resulting MgO materials indicted that the morphology of the precursor hydroxide was retained. The MgO materials were then used as catalysts for Knoevenagel condensation of benzaldehyde and ethyl cyanoacetate. The activity of the MgO samples was found to correspond to the surface hydroxyl density; higher activities (ca. 18 molprod kg−1 h−1) were observed for catalysts with a surface hydroxyl density of >1.3 mmol g−1. Knoevenagel condensation relies greatly on distribution of neighbouring –Mg2+ and –O2− sites, the catalysts with a higher density of potentially low coordination –O2− sites resulted in higher product yields. The hydroxyl density was found to be independent of surface area, such that the combination of precipitating agent and surfactant can effectively control crystal growth to tailor the basic active surface of MgO.

 Please wait while we load your content...
Please wait while we load your content...