Enhanced activity of H2O2-treated copper(ii) oxide nanostructures for the electrochemical evolution of oxygen†
Abstract
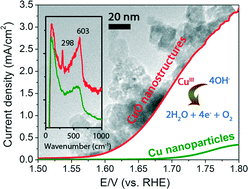
The successful design and synthesis of earth-abundant and efficient catalysts for the oxygen evolution reaction (OER) will be a major step forward towards the use of electrochemical water splitting as an environmentally-friendly process for producing H2 fuel. Due to their poor activity, copper-based materials have not been considered apt for catalysing OER. In this work, we demonstrate that unique copper(II) oxide nanostructures obtained via hydrothermal synthesis and subsequent hydrogen peroxide treatment exhibit unusually high and sustainable OER activity. In 0.1 M KOH electrolyte, the CuO nanostructures catalyse OER with current densities of 2.6–3.4 mA cm−2 at 1.75 V (vs. RHE). The calculated turnover frequency (per Cu site) of ~2 × 10−3 s−1 for O2 production is markedly higher than that of high-surface area electrodeposited Cu metal nanoparticles by 40–68 times. The OER activity of the CuO nanostructures is also stable, approaching about half of 20% IrOx/Vulcan XC-72 after an hour-long OER. In situ Raman spectroscopy at OER-relevant potentials recorded compelling evidence that CuIII active species may be responsible for the unusual OER activity of the CuO nanostructures, as indicated by its signature vibration at 603 cm−1. This hitherto unobserved peak is assigned, with the aid of the model compound NaCuIIIO2, to the Cu–O stretching vibration of CuIII oxide. This feature was not found on electrodeposited Cu metal, which exhibited correspondingly weaker OER activity. The enhanced catalysis of O2 evolution by the CuO nanostructures is thus attributed to not just their higher surface area, but also the higher population of CuIII active sites on their surface.
- This article is part of the themed collection: 2016 most accessed Catalysis Science and Technology articles

 Please wait while we load your content...
Please wait while we load your content...