Selective catalytic reduction of NO with NH3 over novel iron–tungsten mixed oxide catalyst in a broad temperature range†
Abstract
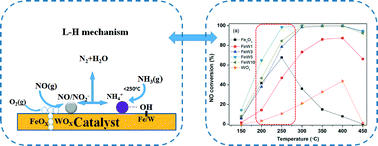
A series of novel FeW(x) catalysts with different Fe/W molar ratios prepared by the co-precipitation method were investigated in the selective catalytic reduction (SCR) of NOx with NH3. Among the tested catalysts, FeW(5) shows the highest catalytic activity, with nearly 100% NO conversion, N2 selectivity from 250 to 450 °C, and good H2O/SO2 durability above 300 °C. After the introduction of WOx species, the lattice structure change to hematite phase and high dispersion of WOx on the surface of the catalysts are observed for the FeW(x) catalysts. The abundance of surface adsorbed oxygen arises from charge imbalance and the formation of unsaturated chemical bonds arising from the interaction between iron oxide species and tungsten oxide species. The Brønsted acid sites are mainly supplied by the W–OH bond, while the Lewis acid sites are related to iron oxide species. Meanwhile the addition of WOx not only leads to the formation of lower-valent iron species, consequently decreasing the Lewis acidity and thereby improving N2 selectivity, but also enhances the stability of surface acid sites and adsorption of monodentate nitrite and NO2 species, which accounts for the excellent catalytic performance of FeW(5) at low temperature. According to the reaction details, Brønsted acid sites play a leading role at low temperature and Lewis acid sites may be the major active centers at high temperature in the SCR process for FeW(x) catalysts.

 Please wait while we load your content...
Please wait while we load your content...