Hydrogen production by the water-gas shift reaction using CuNi/Fe2O3 catalyst†
Abstract
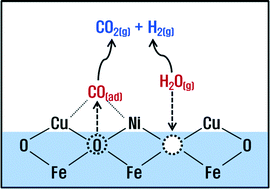
Incorporation of both Cu and Ni together into the crystalline lattice of Fe2O3 results in a significant increase in the catalytic activity and also suppresses the methanation reaction in the high-temperature water-gas shift (HT-WGS) reaction. CuNi/Fe2O3 exhibited the highest CO conversion with negligible CH4 selectivity at the extremely high GHSV of 101 000 h−1 (XCO = 85% at 400 °C). The high activity of CuNi/Fe2O3 catalyst is mainly due to the increase in the lattice strain and the decrease in the binding energy of lattice oxygen. In addition, X-ray photoelectron spectroscopy (XPS) results provide direct evidence for the formation of surface CuNi alloy, which plays a critical role in suppressing the methanation reaction. The detailed characterization by powder X-ray diffraction (XRD), XPS, BET, and H2 temperature-programmed reduction (TPR) techniques was used to understand the role of dopants on host iron oxides in the enhancement of catalytic activity for HT-WGS reaction.

 Please wait while we load your content...
Please wait while we load your content...