Heterogeneous double-activation catalysis: Rh complex and tertiary amine on the same solid surface for the 1,4-addition reaction of aryl- and alkylboronic acids†
Abstract
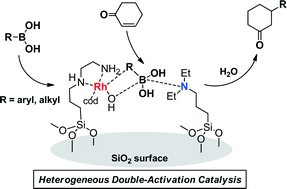
Double-activation catalysis by a rhodium complex/tertiary amine catalyst for the 1,4-addition of organoboronic acids was investigated. A rhodium complex and a tertiary amine were co-immobilized on the same silica surface by silane-coupling reactions followed by complexation of the Rh species. Structures of the Rh complex and tertiary amine on the silica surface were determined by solid-state 13C and 29Si MAS NMR, XAFS, and XPS measurements. The immobilized tertiary amine accelerated the Rh-catalyzed 1,4-addition of phenylboronic acid to cyclohexenone. The role of the anchored tertiary amine in the 1,4-addition reaction was clarified by solid-state 11B MAS NMR: it activates the arylboronic acid forming a four-coordinate boron species, which then accelerates the transmetallation step in the Rh complex-catalyzed 1,4-addition. The silica-supported Rh complex/tertiary amine catalyst system could be applicable to the reaction of various aryl- and even alkyl-boronic acids.

 Please wait while we load your content...
Please wait while we load your content...