Continuous synthesis of diethyl carbonate from ethanol and CO2 over Ce–Zr–O catalysts
Abstract
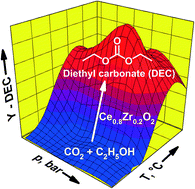
CexZr1−xO2 (x = 0, 0.2, 0.5, 0.8 and 1.0) solids were prepared by a citrate method and characterized by various techniques such as N2-adsorption (BET-SA), XRD, XPS, TEM, H2-TPR, NH3- and CO2-TPD. The catalytic performance of these solids was evaluated for the direct synthesis of diethyl carbonate (DEC) from ethanol and CO2 in continuous mode using a plug-flow reactor (PFR). According to thermodynamic data, the reaction is favourable at low reaction temperatures and high reaction pressures. Thus, the catalytic experiments were carried out at reaction temperatures ranging from 80 to 180 °C and at reaction pressures from 80 to 180 bar. The CexZr1−xO2 catalysts exhibited significant differences in their performance mainly depending on (i) their Ce : Zr ratio and (ii) the different acid–base characteristics. Among the series Ce0.8Zr0.2O2 (C80Z) and Ce0.5Zr0.5O2 (C50Z) catalysts displayed the most efficient performance. Moreover, C80Z, pretreated at 700 °C, yielded DEC at the equilibrium conversion level of YDEC ~ 0.7% at 140 °C and 140 bar at a CO2 : ethanol ratio of 6 : 1 at a LHSV of 42 Lliq kgcat−1 h−1.

 Please wait while we load your content...
Please wait while we load your content...