Nanoscale metal fluorides: a new class of heterogeneous catalysts†
Abstract
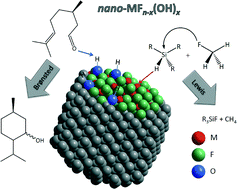
This perspective article focuses on nanoscopic metal fluorides and hydroxide fluorides prepared via a recently explored fluorolytic sol–gel synthesis approach. Metal fluoride phases obtained via this route exhibit distinctly different properties compared with their classically prepared homologues. Thus, extremely strong solid Lewis acids are available which give access to new catalytic reactions with sometimes unexpectedly high conversion degrees and selectivity. Even more interestingly, metal hydroxide fluorides can be obtained via this synthesis route, which are not accessible via any other approach for which the hydroxide to fluoride ratio can be adjusted over a wide range. As a result, bi-acidic (Brønsted and Lewis) solids with tunable Lewis to Brønsted acidity can be obtained which show interesting results in a variety of reactions. Finally, these new nano-metal fluorides, due to their very high surface areas and distinct acidic properties, can be used as supports for many novel metal catalysed reactions, thus showing surprising synergistic effects. This overview will briefly outline the synthesis approach of the fluorolytic sol–gel route, will present characteristic bulk and surface properties and will give several examples of their novel catalytic applications in purely Lewis acid and in bi-acidic catalysed reactions, and will also exemplarily show the potential of these new materials as supports for heterogeneous catalytic reactions.

 Please wait while we load your content...
Please wait while we load your content...