Ruthenium(ii)-catalyzed switchable C3-alkylation versus alkenylation with acrylates of 2-pyridylbenzofurans via C–H bond activation†
Abstract
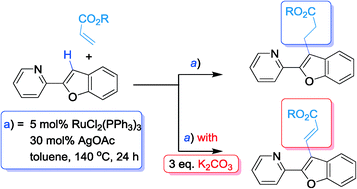
We documented an interesting observation of ruthenium(II)-catalyzed benzofuran C–H activation and subsequent functionalization with acrylates that reveals that a simple base can switch the process from alkylation to alkenylation.

 Please wait while we load your content...
Please wait while we load your content...