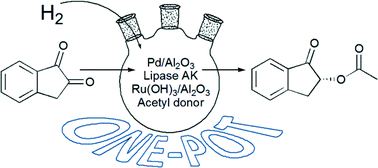
One-pot synthesis of (R)-2-acetoxy-1-indanone from 1,2-indanedione combining metal catalyzed hydrogenation and chemoenzymatic dynamic kinetic resolution†
Abstract
Combination of the regioselective hydrogenation of the prochiral diketone 1,2-indanedione with chemoenzymatic dynamic kinetic resolution of the resulting rac-2-hydroxy-1-indanone was investigated. A new simple to operate, one-pot reaction sequence provides the valuable building block (R)-2-acetoxy-1-indanone in moderate enantiopurity (86–92% ee) and a competitive isolated yield (39%) compared with a traditional isolated batch reaction approach.

 Please wait while we load your content...
Please wait while we load your content...