Efficient and recyclable Cu2(BDC)2(BPY)-catalyzed oxidative amidation of terminal alkynes: role of bipyridine ligand†
Abstract
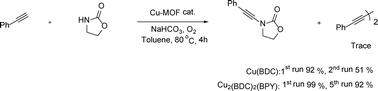
We have described an efficient method for oxidative cross coupling reactions between activated N–H amines and terminal alkynes using heterogeneous Cu2(BDC)2(BPY) as recyclable catalyst (BDC = benzene-1,4-dicarboxylate; BPY = 4,4′-bipyridine). The optimal conditions involved the use of inexpensive NaHCO3 base and oxygen as terminal oxidant in toluene solvent at 80 °C. Reactions proceeds efficiently, and high selectivity with only trace amount of diynes as well as good yields were achieved in a short reaction time. The Cu2(BDC)2(BPY) exhibited excellent catalytic activity and selectivity as compared to other Cu-MOFs on a broad reaction scope. Interestingly, the presence of bipyridine ligand was shown to enhance the catalyst stability. Thus, the Cu2(BDC)2(BPY) could be facilely separated from the reaction mixture by simple centrifugation and could be reused several times with only a slight decrease in catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...