TiO2-supported heteropoly acid catalysts for dehydration of methanol to dimethyl ether: relevance of dispersion and support interaction†
Abstract
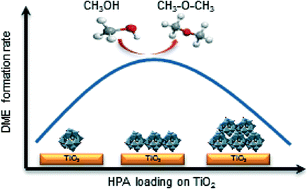
Two heteropoly acids (HPAs) with Keggin structures, namely, H3PW12O40 (HPW) and H4SiW12O40 (HSiW), have been deposited on TiO2 and used as catalysts for the production of dimethyl ether (DME) from methanol. The catalysts have been prepared by incipient wetness impregnation of HPW and HSiW on TiO2 with HPA loadings ranging between 0.9 and 9.0 Keggin units (KU) per square nanometer. The structure and acid properties of the final catalysts have been thoroughly characterized by N2 adsorption–desorption isotherms, TGA, XRD, Raman spectroscopy, XPS, NH3 adsorption isotherms, DRIFT and 1H NMR. All catalysts exhibit very high DME productivities and high methanol conversion rates at temperatures as low as 413 K. The effect of the HPA loading on TiO2 for the production of DME has been correlated with the structure and acid properties of the final catalyst. We find an optimum loading for both TiO2-supported HPW and HSiW of 2.3 KU nm−2. At this level, both HPW and HSiW are well dispersed onto the support, thus permitting the access of methanol to the active acid sites. On the contrary, higher HPA loadings on TiO2 result in the formation of larger HPA units that prevent the access of methanol to the inner acid sites within the HPA. On the other hand, HPA loadings below 2.3 KU nm−2 result in a strong interaction between the acid protons of the HPAs and the support, hence preventing the participation of such protons in the methanol dehydration reaction, which results in lower methanol conversion rates.

 Please wait while we load your content...
Please wait while we load your content...