Cyclopentadienyl molybdenum alkyl ester complexes as catalyst precursors for olefin epoxidation†
Abstract
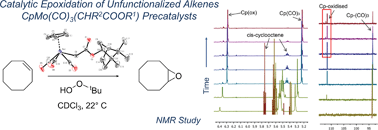
New molybdenum complexes of the type [CpMo(CO)3X] containing ligands of the formula X = CHR2CO(OR1) where R1 = ethyl (1), menthyl (4), and bornyl (5) and R2 = H; R1 = ethyl and R2 = methyl (2) and phenyl (3) have been synthesized and characterized by NMR and IR spectroscopy and X-ray crystallography. These compounds have been applied as catalyst precursors for achiral and chiral epoxidation of unfunctionalized olefins with tert-butyl hydroperoxide (TBHP) as the oxidant at 22 °C (in CH2Cl2) and 55 °C (in CHCl3). The substrates cis-cyclooctene, 1-octene, cis- and trans-stilbene, and trans-β-methylstyrene were selectively and quantitatively converted into their epoxides using a catalyst : substrate : oxidant ratio of 1 : 100 : 200 within 4 h at room temperature in CH2Cl2 and within 15 min at 55 °C in CHCl3. Complexes 1–5 are precursors of active epoxidation catalysts and turnover frequencies (TOFs) of ca. 1200 h−1 are obtained with cis-cyclooctene as the substrate. No enantioselectivity is observed with trans-β-methylstyrene as the substrate despite the application of enantiomerically pure precatalysts. In situ monitoring of catalytic epoxidation of cis-cyclooctene with complex 5 by 1H and 13C NMR spectroscopy suggests that the chiral alkyl ester side chain is retained during oxidation with TBHP. During epoxidation, the primary catalytic species is the dioxo complex [CpMoO2X]. After near complete conversion of cis-cyclooctene to its epoxide, further oxidation of the dioxo complex to oxo–peroxo complex [CpMo(η2-O2)(O)X] takes place. The oxo–peroxo complex is also an active epoxidation catalyst.

 Please wait while we load your content...
Please wait while we load your content...