The migration of Cu species over Cu–SAPO-34 and its effect on NH3 oxidation at high temperature†
Abstract
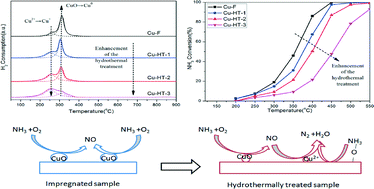
The influence of Cu species migration, through hydrothermal treatment, on the NH3 oxidation mechanism and its inhibition on NH3-SCR activity over Cu–SAPO-34 catalysts were studied. XRD, SEM, EPR, H2-TPR, CO-DRIFTS were conducted to estimate the Cu species distribution. The results revealed that the CuO species initially existed on the external surface of the fresh impregnated Cu/SAPO-34 catalyst, and converted to isolated Cu2+ and nanosized CuO in the cavity of the SAPO-34 support during hydrothermal treatment. The NH3-SCR activity over the hydrothermally treated Cu/SAPO-34 catalysts improved due to the increase of the isolated Cu2+ amount, while the NH3 oxidation activity declined. Furthermore, the NO amount generated in the NH3 oxidation reaction over the hydrothermally treated samples was much less than the fresh one, which was also caused by the migration of Cu species. In addition, the kinetic NH3 oxidation was performed at temperatures from 340 °C to 440 °C, and the results indicated that CuO species were the active sites for the NH3 oxidation. In conclusion, the Cu species migration caused the decrease of the NH3 oxidation activity and the great variation in the generated NO amount. Furthermore, it was proposed that the NH3 oxidation mechanism over the Cu/SAPO-34 catalysts contained two steps: firstly, molecular NH3 reacted with O2 at CuO sites; secondly, the generated NO was reduced by NH3 to N2 at isolated Cu2+ sites.

 Please wait while we load your content...
Please wait while we load your content...