Catalytic hydrosilylation of oxalic acid: chemoselective formation of functionalized C2-products†
Abstract
Oxalic acid is an attractive entry to functionalized C2-products because it can be formed by C–C coupling of two CO2 molecules under electrocatalytic reduction. Herein, we describe the first attempts to reduce oxalic acid by catalytic hydrosilylation. Using B(C6F5)3 as a Lewis acidic catalyst, oxalic acid can be converted to reduced C2-molecules, with high chemoselectivity, under mild reaction conditions.
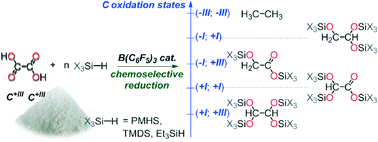
- This article is part of the themed collection: Sustainable catalytic conversions of renewable substrates

 Please wait while we load your content...
Please wait while we load your content...