Effect of post-synthesis hydrogen-treatment on the nature of iron species in Fe-BEA as NH3-SCR catalyst
Abstract
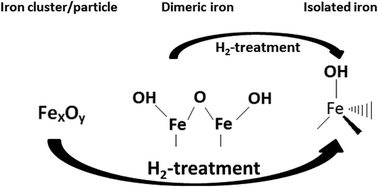
Post-synthesis treatment of Fe-BEA with hydrogen has previously been shown to improve the low-temperature activity for NO reduction during standard NH3-SCR. Here, a 2 wt.% Fe-BEA sample was prepared by incipient wetness impregnation and calcined in air at 450 °C for 3 h. The fresh sample was then treated with 5% H2 at 650 °C for 5 h. The evolution of different iron species in Fe-BEA before and after H2-treatment was studied using in situ DRIFT spectroscopy with NO as a probe molecule and by UV-Vis spectroscopy. The DRIFTS results show that the relative intensity of the absorption peak representing isolated iron species increases significantly after H2-treatment of the fresh Fe-BEA sample. Furthermore, the UV-Vis results show a significant decrease in the relative intensity in the UV region representing larger iron particles, whereas the relative intensity representing smaller iron species increases after treatment of Fe-BEA with hydrogen. The results show that the low-temperature NO reduction during standard NH3-SCR can be increased for Fe-BEA by redispersion of smaller iron species into the zeolite structure by hydrogen-treatment.

 Please wait while we load your content...
Please wait while we load your content...