Tetrazole amides as hydrogen-bonding donor catalysts in the chemoselective oxidation of sulphides and disulphides†
Abstract
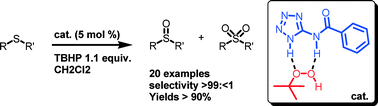
The oxidation of organosulphides catalyzed by hydrogen bonding donors deriving from aminotetrazole has been studied. The oxidation reaction was performed in CH2Cl2 solution using TBHP (1.1 eq.) as a versatile and chemoselective new catalyst to sulfoxides. 5 mol.% catalyst loading afforded organosulfoxides with complete conversion and yields around 90–95%. Tetrazole amide derivatives can be easily recovered by simple filtration and reused several times. Reactions were carried out in scales ranging between mg and multigram in order to test the robustness of the process. 1H-NMR studies and DFT calculations were exploited to disclose the role of tetrazole amide–TBHP complexes in the sulphides oxidation reaction as new performing catalysts.

 Please wait while we load your content...
Please wait while we load your content...