Hydrothermal synthesis of zinc indium sulfide microspheres with Ag+ doping for enhanced H2 production by photocatalytic water splitting under visible light†
Abstract
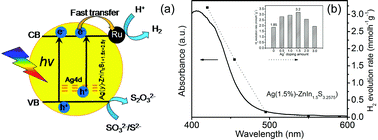
Two series of ZnInxS1+1.5x and Ag(y)–ZnInxS1+1.5x+0.5y solid solutions are prepared by hydrothermal methods. The synthetic conditions such as the molar ratio of In/Zn, the pH value, the hydrothermal temperature and the reaction time are found to intensely influence the crystal structure and the morphology of the photocatalyst as well as its photocatalytic activity for H2 generation from water. It is revealed that the ZnIn1.5S3.25 solid solution (In/Zn = 1.5) prepared at 160 °C for 6 h by adding 1 mL hydrochloric acid to the precursor solution shows the highest photocatalytic H2 evolution rate of 1.85 mmol h−1 g−1 in the presence of Ru as the co-catalyst and Na2S/Na2SO3 as sacrificial reagents. Furthermore, after Ag+ doping, the photocatalytic H2 evolution rate remarkably increased to 3.20 mmol h−1 g−1 for the Ag(1.5%)–ZnIn1.5S3.2575 sample. This work provides a new opportunity to develop efficient photocatalysts for photosplitting water into hydrogen.

 Please wait while we load your content...
Please wait while we load your content...