Molten copper hexaoxodivanadate: an efficient catalyst for SO3 decomposition in solar thermochemical water splitting cycles†
Abstract
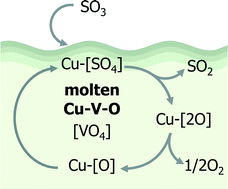
Molten copper hexaoxodivanadate (CuV2O6) was identified as an active catalyst for SO3 decomposition, which is an oxygen generation step in solar thermochemical water splitting cycles, at moderate temperatures (ca. 600 °C). The SO3 decomposition over CuV2O6 was significantly accelerated when the reaction temperature approached the melting point (ca. 630 °C) compared with solid phases of Cu2V2O7 as well as other compounds in the CuO–V2O5 system with higher melting points (≥780 °C). A possible intermediate CuSO4 species formed by SO3 adsorption onto the Cu oxide site may decompose promptly to evolve SO2 and O2 on contact with the molten catalyst phase. Furthermore, the molten catalyst contained a large fraction of monovalent Cu formed by spontaneous desorption of oxygen. A possible reaction mechanism consisting of the fast dissolution of CuSO4 and Cu2+/Cu+ redox cycles in the melt is proposed.

 Please wait while we load your content...
Please wait while we load your content...