Competition of selective catalytic reduction and non selective catalytic reduction over MnOx/TiO2 for NO removal: the relationship between gaseous NO concentration and N2O selectivity†
Abstract
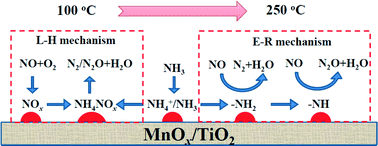
In this work, a novel phenomenon was discovered that N2O selectivity of NO reduction over MnOx/TiO2 was related to the concentration of gaseous NO and that lower concentration of gaseous NO would cause higher N2O selectivity. In situ DRIFTS and transient reaction studies demonstrated that both the Eley–Rideal mechanism (the reaction of over-activated NH3 with gaseous NO) and the Langmuir–Hinshelwood mechanism (the reaction of adsorbed NO3− with adsorbed NH3 on the adjacent sites) could contribute to the formation of N2O. Kinetic study demonstrated that N2O selectivity would be independent of gaseous NO concentration if NO reduction over MnOx/TiO2 mainly followed the Langmuir–Hinshelwood mechanism. If NO reduction over MnOx/TiO2 mainly followed the Eley–Rideal mechanism, there was competition between the selective catalytic reduction (SCR) reaction and non selective catalytic reduction (NSCR) reaction. As gaseous NO concentration increased, more –NH2 was used to reduce gaseous NO to form N2 and the further oxidization of –NH2 to –NH was restrained, resulting in an obvious decrease of N2O selectivity. The Eley–Rideal mechanism played an important role in NO reduction over MnOx/TiO2, especially at higher temperatures. Therefore, N2O selectivity of the low temperature SCR reaction over MnOx/TiO2 decreased especially at higher temperatures after the increase of gaseous NO concentration.

 Please wait while we load your content...
Please wait while we load your content...