Intercorrelation of structure and performance of Ni–Mg/Al2O3 catalysts prepared with different methods for syngas methanation
Abstract
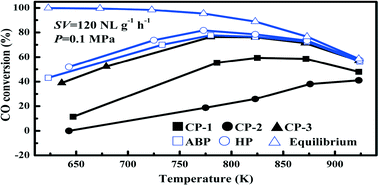
Ni–Mg/Al2O3 catalysts prepared with methods of co-precipitation (CP), homogeneous precipitation (HP) and acid–base pairing (ABP) were tested for syngas methanation at 623–923 K in a stainless steel fixed bed reactor and further characterized to justify their performances. The Ni–Mg/Al2O3 catalysts prepared with CP using NH4OH, NaOH and Na2CO3 as the precipitants followed an activity order of NaOH > NH4OH > Na2CO3. Comparing further with the samples prepared by HP and ABP resulted in an activity order of HP > ABP > CP (NaOH) for syngas methanation under 0.1 MPa. For CO conversion, a transition of reaction control from kinetic dominance to thermodynamic dominance was observed at about 780 K. Performing 20 h continuous tests at 773 K and under 0.1 and 2.5 MPa further verified the stability of the HP, ABP and CP (NaOH) catalysts for syngas methanation. Analyzing the catalysts via H2-TPR clarified that a lower amount of free NiO and a stronger interaction between the dispersed NiO and Al2O3 or MgO ensured better catalytic performance for methanation. The study also identified two types of carbon deposited on the surface of the spent catalysts.

 Please wait while we load your content...
Please wait while we load your content...