Physical mixing of metal acetates: optimisation of catalyst parameters to produce highly active bimetallic catalysts
Abstract
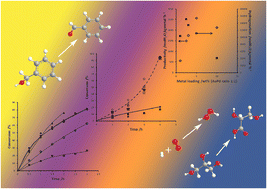
We have investigated the optimisation of the catalytic parameters for the preparation of
- This article is part of the themed collection: Gold Catalysis

 Please wait while we load your content...
Please wait while we load your content...