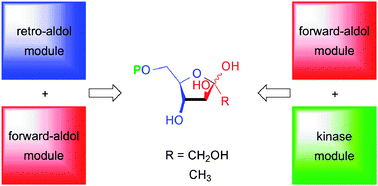
We have evaluated different strategies for the one-pot synthesis of D-fructose 6-phosphate and its 1-deoxy and 3-deoxy analogs from readily available starting materials, by building up “artificial metabolisms” in vitro. The first consisted of an aldol cleavage–aldol formation cascade, in which glyceraldehyde 3-phosphate as the central intermediate is generated from fructose 1,6-bisphosphate and consumed in situ for a consecutive carboligation step catalyzed by fructose 6-phosphate aldolase (FSA). The second approach consisted of an aldolase–kinase coupling, in which the unphosphorylated ketose was produced in situ by FSA-catalyzed carboligation, followed by a hexokinase-catalyzed phosphorylation step. While both approaches profited from the high stereoselectivity and stability of the aldolases used, the first approach proved to be the most practical, effective and economical, whereas the second strongly depends on the substrate specificity of hexokinase, which shows inferior catalytic efficiency with the 1-deoxy substrate. Phosphorylation of 3-deoxyfructose failed because, contradictory to a literature report, this compound was found not to be acceptable as a substrate of yeast hexokinase.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...