On the rate-determining step and the ligand electronic effects in rhodium catalysed hydrogenation of enamines and the hydroaminomethylation of alkenes†
Abstract
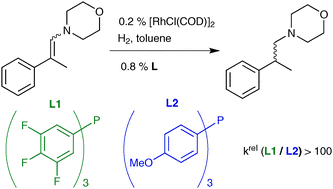
In the course of studies on the tandem hydroformylation–reductive amination (hydroaminomethylation), fluorinated mono-phosphines were found to be more active than their more electron-donating counterparts in the

 Please wait while we load your content...
Please wait while we load your content...