Understanding the role of thermodynamics in catalytic imine reductions†
Abstract
The past decade has witnessed a booming growth of research activity in catalytic imine reduction, due to the ongoing motivation to find more efficient conversions of the rather unreactive C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds to the desired C–N segments found in many pharmaceuticals. While several timely reviews have well documented various C
N bonds to the desired C–N segments found in many pharmaceuticals. While several timely reviews have well documented various C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N reduction methodologies with respect to the type of catalyst (acid, base, or transition metal), a detailed discussion of the core role of thermodynamic driving forces in governing these catalyses is still lacking, however. Hence, this tutorial review describes some of the most practical considerations for adjusting reduction thermodynamics by choosing appropriate catalytic strategies, in order to make the target reduction energetically feasible. The combined use of relevant thermodynamic parameters of the substrate imines, hydrogen donors, and catalysts in realizing such a goal is demonstrated on the basis of the energetics of the possible elementary paths. Experimental observations from the literature that are in line with the present energetics-based analyses are exemplified.
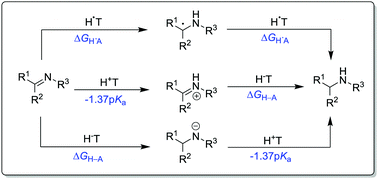
N reduction methodologies with respect to the type of catalyst (acid, base, or transition metal), a detailed discussion of the core role of thermodynamic driving forces in governing these catalyses is still lacking, however. Hence, this tutorial review describes some of the most practical considerations for adjusting reduction thermodynamics by choosing appropriate catalytic strategies, in order to make the target reduction energetically feasible. The combined use of relevant thermodynamic parameters of the substrate imines, hydrogen donors, and catalysts in realizing such a goal is demonstrated on the basis of the energetics of the possible elementary paths. Experimental observations from the literature that are in line with the present energetics-based analyses are exemplified.


 Please wait while we load your content...
Please wait while we load your content...