Catalysis for the synthesis of methacrylic acid and methyl methacrylate†
Abstract
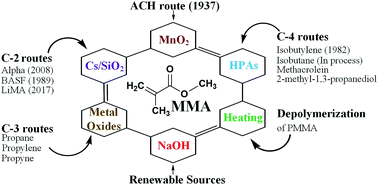
Methyl methacrylate (MMA) is a specialty monomer for poly methyl methacrylate (PMMA) and the increasing demand for this monomer has motivated industry to develop clean technologies based on renewable resources. The dominant commercial process reacts acetone and hydrogen cyanide to MMA (ACH route) but the intermediates (hydrogen cyanide, and acetone cyanohydrin) are toxic and represent an environmental hazard. Esterification of methacrylic acid (MAA) to MMA is a compelling alternative together with ethylene, propylene, and isobutene/t-butanol as feedstocks. Partially oxidizing isobutane or 2-methyl-1,3-propanediol (2MPDO) over heteropolycompounds to MAA in a single-step is nascent technology to replace current processes. The focus of this review is on catalysts and their role in the development of processes herein described. Indeed, in some cases remarkable catalysts were studied that enabled considerable steps forward in both the advancement of catalysis science and establishing the basis for new technologies. An emblematic example is represented by Keggin-type heteropolycompounds with cesium and vanadium, which are promising catalysts to convert isobutane and 2MPDO to MAA. Renewable sources for the MMA or MAA route include acetone, isobutanol, ethanol, lactic, itaconic, and citric acids. End-of-life PMMA is expected to grow as a future source of MMA.


 Please wait while we load your content...
Please wait while we load your content...