Catalytic nucleophilic ‘umpoled’ π-allyl reagents
Abstract
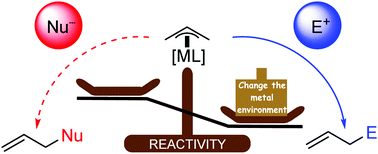
After seminal Tsuji–Trost reactions (palladium catalyzed allylation of nucleophiles via π-allyl intermediates as electrophiles), the idea of reversal reactivity of π-allyl intermediates (i.e. π-allyl as nucleophiles) has been stated since the eighties. Thanks to different transition metal sources and the modification of their electronic environment through the use of additives and ligands, such ‘reactivity switch’ of π-allyl intermediates proved its powerfulness allowing high control in regio-, diastereo- and enantio-selectivities. These methodologies have thus emerged as efficient methods in the catalytic enantioselective allylation of carbonyl compounds and imines with a deep impact on natural product and/or drug elaboration. This tutorial review highlights the concept of ‘umpoled’ reactivity of π-allyl intermediates, relying on selected recent examples.


 Please wait while we load your content...
Please wait while we load your content...