Boron-selective reactions as powerful tools for modular synthesis of diverse complex molecules
Abstract
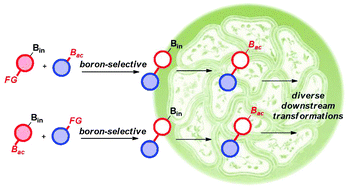
In the context of modular and rapid construction of molecular diversity and complexity for applications in organic synthesis, biomedical and materials sciences, a generally useful strategy has emerged based on boron-selective chemical transformations. In the last decade, these types of reactions have evolved from proof-of-concept to some advanced applications in the efficient preparation of complex natural products and even automated precise manufacturing on the molecular level. These advances have shown the great potential of boron-selective reactions in simplifying synthetic design and experimental operations, and should inspire new developments in related chemical and technological areas. This tutorial review will highlight the original contributions and representative advances in this emerging field.

 Please wait while we load your content...
Please wait while we load your content...