Progress in asymmetric biomimetic transamination of carbonyl compounds
Abstract
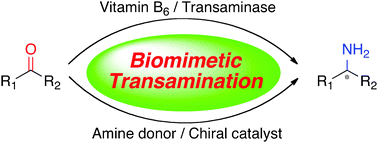
Transamination of α-keto acids with transaminases and pyridoxamine phosphate is an important process to form optically active α-amino acids in biological systems. Various biomimetic transamination systems have been developed for carbonyl compounds including α-keto acid derivatives, fluoroalkyl ketones, and unactivated ketones with chiral vitamin B6 analogues, artificial transaminase mimics, chiral nitrogen sources, and chiral catalysts. This review provides a brief summary of this area.

 Please wait while we load your content...
Please wait while we load your content...