Crucial aspects in the design of chirally modified noble metal catalysts for asymmetric hydrogenation of activated ketones
Abstract
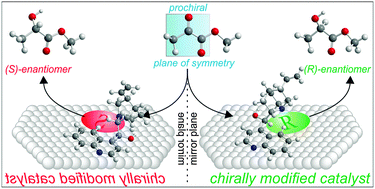
In view of the importance of optically pure chiral products there is ample reason to develop methods that facilitate their efficient production. Compared to the mostly applied homogeneous catalysts based on transition metals coordinated to suitable chiral ligands, heterogeneous chiral catalysts could offer several features that are beneficial for practical application such as stability, ease of catalyst separation and regeneration as well as straightforward access to continuous process operation. Various strategies have been developed for imparting chirality to catalytic active surfaces, among which the chiral modification of active metal surfaces by adsorption of suitable chiral organic compounds has so far been among the most successful. In this tutorial review lessons learned from research on asymmetric hydrogenation on chirally modified noble metals will be presented. Key aspects for the design of such catalysts will be elucidated using chirally modified platinum catalysts for the asymmetric hydrogenation of α-activated ketones as an example.

 Please wait while we load your content...
Please wait while we load your content...