Hydrogen bonding in ionic liquids†
Abstract
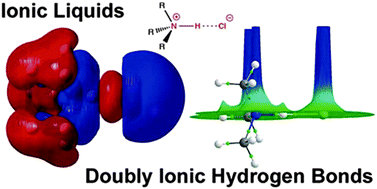
Ionic liquids (IL) and hydrogen bonding (H-bonding) are two diverse fields for which there is a developing recognition of significant overlap. Doubly ionic H-bonds occur when a H-bond forms between a cation and anion, and are a key feature of ILs. Doubly ionic H-bonds represent a wide area of H-bonding which has yet to be fully recognised, characterised or explored. H-bonds in ILs (both protic and aprotic) are bifurcated and chelating, and unlike many molecular liquids a significant variety of distinct H-bonds are formed between different types and numbers of donor and acceptor sites within a given IL. Traditional more neutral H-bonds can also be formed in functionalised ILs, adding a further level of complexity. Ab initio computed parameters; association energies, partial charges, density descriptors as encompassed by the QTAIM methodology (ρBCP), qualitative molecular orbital theory and NBO analysis 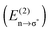 provide established and robust mechanisms for understanding and interpreting traditional neutral and ionic H-bonds. In this review the applicability and extension of these parameters to describe and quantify the doubly ionic H-bond has been explored. Estimating the H-bonding energy is difficult because at a fundamental level the H-bond and ionic interaction are coupled. The NBO and QTAIM methodologies, unlike the total energy, are local descriptors and therefore can be used to directly compare neutral, ionic and doubly ionic H-bonds. The charged nature of the ions influences the ionic characteristics of the H-bond and vice versa, in addition the close association of the ions leads to enhanced orbital overlap and covalent contributions. The charge on the ions raises the energy of the Ylp and lowers the energy of the X–H σ* NBOs resulting in greater charge transfer, strengthening the H-bond. Using this range of parameters and comparing doubly ionic H-bonds to more traditional neutral and ionic H-bonds it is clear that doubly ionic H-bonds cover the full range of weak through to very strong H-bonds.
provide established and robust mechanisms for understanding and interpreting traditional neutral and ionic H-bonds. In this review the applicability and extension of these parameters to describe and quantify the doubly ionic H-bond has been explored. Estimating the H-bonding energy is difficult because at a fundamental level the H-bond and ionic interaction are coupled. The NBO and QTAIM methodologies, unlike the total energy, are local descriptors and therefore can be used to directly compare neutral, ionic and doubly ionic H-bonds. The charged nature of the ions influences the ionic characteristics of the H-bond and vice versa, in addition the close association of the ions leads to enhanced orbital overlap and covalent contributions. The charge on the ions raises the energy of the Ylp and lowers the energy of the X–H σ* NBOs resulting in greater charge transfer, strengthening the H-bond. Using this range of parameters and comparing doubly ionic H-bonds to more traditional neutral and ionic H-bonds it is clear that doubly ionic H-bonds cover the full range of weak through to very strong H-bonds.

 Please wait while we load your content...
Please wait while we load your content...