Functional group directed C–H borylation†
Abstract
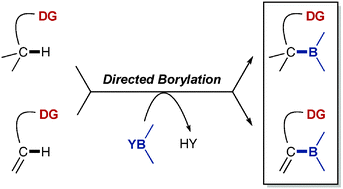
The direct borylation of hydrocarbons via C–H activation has reached an impressive level of sophistication and efficiency, emerging as a fundamental tool in synthesis because of the versatility offered by organoboron compounds. As a remarkable particularity, the catalytic systems originally developed for these reactions are relatively insensitive to directing effects, and the regioselectivity of the borylations is typically governed by steric factors. Likely stimulated by the great synthetic potential of the expected functionalised organoboranes, however, many groups have recently focused on the development of complementary strategies for directed, site-selective borylation reactions where a directing group controls the course of the reaction. In this tutorial review, the different strategies and findings related to the development of these directed borylation reactions via C(sp2)–H or C(sp3)–H activation will be summarized and discussed.

 Please wait while we load your content...
Please wait while we load your content...