Beyond click chemistry – supramolecular interactions of 1,2,3-triazoles
Abstract
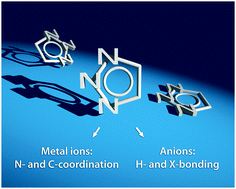
The research on 1,2,3-triazoles has been lively and ever-growing since its stimulation by the advent of click chemistry. The attractiveness of 1H-1,2,3-triazoles and their derivatives originates from their unique combination of facile accessibility via click chemistry and truly diverse supramolecular interactions, which enabled myriads of applications in supramolecular and coordination chemistry. The nitrogen-rich triazole features a highly polarized carbon atom allowing the complexation of anions by hydrogen and halogen bonding or, in the case of the triazolium salts, via charge-assisted hydrogen and halogen bonds. On the other hand, the triazole offers several N-coordination modes including coordination via anionic and cationic nitrogen donors of triazolate and triazolium ions, respectively. After CH-deprotonation of the triazole and the triazolium, powerful carbanionic and mesoionic carbene donors, respectively, are available. The latter coordination mode even features non-innocent ligand behavior. Moreover, these supramolecular interactions can be combined, e.g., in ion-pair recognition, preorganization by intramolecular hydrogen bond donation and acceptance, and in bimetallic complexes. Ultimately, by clicking two building blocks into place, the triazole emerges as a most versatile functional unit allowing very successful applications, e.g., in anion recognition, catalysis, and photochemistry, thus going far beyond the original purpose of click chemistry. It is the intention of this review to provide a detailed analysis of the various supramolecular interactions of triazoles in comparison to established functional units, which may serve as guidelines for further applications.

 Please wait while we load your content...
Please wait while we load your content...