Catalytic amide formation from non-activated carboxylic acids and amines
Abstract
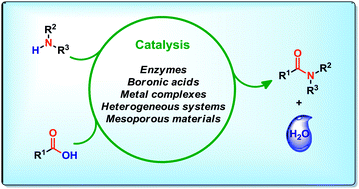
The amide functionality is found in a wide variety of biological and synthetic structures such as proteins, polymers, pesticides and pharmaceuticals. Due to the fact that synthetic amides are still mainly produced by the aid of coupling reagents with poor atom-economy, the direct catalytic formation of amides from carboxylic acids and amines has become a field of emerging importance. A general, efficient and selective catalytic method for this transformation would meet well with the increasing demands for green chemistry procedures. This review covers catalytic and synthetically relevant methods for direct condensation of carboxylic acids and amines. A comprehensive overview of homogeneous and heterogeneous catalytic methods is presented, covering biocatalysts, Lewis acid catalysts based on boron and metals as well an assortment of other types of catalysts.

 Please wait while we load your content...
Please wait while we load your content...