Trivalent boron nucleophile as a new tool in organic synthesis: reactivity and asymmetric induction
Abstract
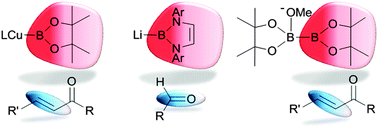
Boron compounds have been traditionally regarded as “Lewis acids” preferring to accept electrons rather than donate them in the course of their reactions but current examples of unusual reactivity between tricoordinated

 Please wait while we load your content...
Please wait while we load your content...