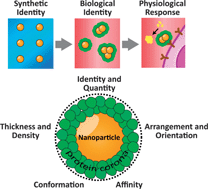
Nanomaterials hold promise as multifunctional diagnostic and therapeutic agents. However, the effective application of nanomaterials is hampered by limited understanding and control over their interactions with complex biological systems. When a nanomaterial enters a physiological environment, it rapidly adsorbs proteins forming what is known as the protein ‘corona’. The protein corona alters the size and interfacial composition of a nanomaterial, giving it a biological identity that is distinct from its synthetic identity. The biological identity determines the physiological response including signalling, kinetics, transport, accumulation, and toxicity. The structure and composition of the protein corona depends on the synthetic identity of the nanomaterial (size, shape, and composition), the nature of the physiological environment (blood, interstitial fluid, cell cytoplasm, etc.), and the duration of exposure. In this critical review, we discuss the formation of the protein corona, its structure and composition, and its influence on the physiological response. We also present an ‘adsorbome’ of 125 plasma proteins that are known to associate with nanomaterials. We further describe how the protein corona is related to the synthetic identity of a nanomaterial, and highlight efforts to control protein–nanomaterial interactions. We conclude by discussing gaps in the understanding of protein–nanomaterial interactions along with strategies to fill them (167 references).

 Please wait while we load your content...
Please wait while we load your content...