Bond formations by intermolecular and intramolecular trappings of acylketenes and their applications in natural product synthesis†
Abstract
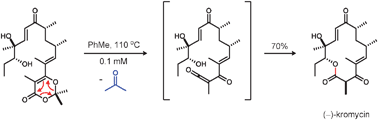
The reactive intermediates known as acylketenes exhibit a rich chemistry and have been extensively utilized for many types of inter- and intramolecular bond-forming reactions within the field of organic synthesis. Characteristic reactions of acylketenes include cycloadditions, carbon–carbon bond-forming reactions, and nucleophilic capture with alcohols or amines to give β-keto acid derivatives. In particular, the intramolecular capture of acylketene intermediates with pendant nucleophiles represents a powerful method for forming both medium-sized rings and macrocycles, often in high yield. This tutorial review examines the history, generation, and reactivity of acylketenes with a special focus on their applications in the synthesis of natural products.
- This article is part of the themed collection: Rapid complexity generation in natural product total synthesis

 Please wait while we load your content...
Please wait while we load your content...