Engineering discrete stacks of aromatic molecules†
Abstract
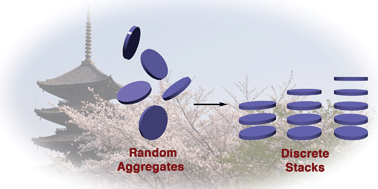
Intrigued by transannular interactions occurring in stacked aromatic molecules, chemists have long endeavored to engineer discrete stacks of specific lengths and orientation. The maturation of
- This article is part of the themed collection: Jean-Pierre Sauvage

 Please wait while we load your content...
Please wait while we load your content...