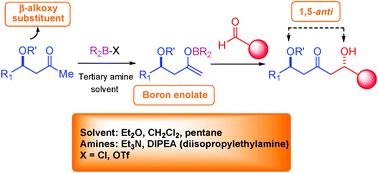
This tutorial review describes that high levels of substrate-controlled, 1,5-stereoinduction are obtained in the boron-mediated aldol reactions of β-oxygenated methyl ketones with achiral and chiral aldehydes. Remote induction from the boron enolates gives the 1,5-anti adducts, with the enolate π-facial selectivity critically dependent upon the nature of the β-alkoxy protecting group. This 1,5-anti aldol methodology has been strategically employed in the total synthesis of several natural products with remarkable pharmacological activities. At present, the origin of the high level of 1,5-anti induction obtained with the boron enolates is unclear, although a model based on hydrogen bonding between the β-alkoxy oxygen and the formyl aldehyde hydrogen has recently been proposed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...