Calorimetric and computational study of sulfur-containing six-membered rings
Abstract
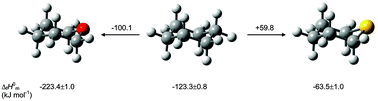
Thermochemical data, and in particular the enthalpies of formation of oxygen- and sulfur-containing six-membered heterocycles provide essential information on the factors responsible for the contrasting behavior (structural, conformational and reactivity) between these types of compounds. A proper understanding of the experimental observations requires theoretical modeling in order to confirm the relative importance of the steric, electronic, electrostatic and stereoelectronic interactions that are responsible of the enthalpies of formation for the

 Please wait while we load your content...
Please wait while we load your content...