Geometrical constraints of thermal dehydration of β-calcium sulfate hemihydrate induced by self-generated water vapor†
Abstract
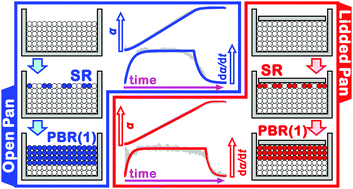
The thermal dehydration of calcium sulfate dihydrate exhibits a complex reaction behavior, in which the reaction pathway and kinetics vary depending on water vapor pressure (p(H2O)) applied as the atmospheric condition and generated in the course of the reaction. Under high p(H2O) conditions, a crystalline hemihydrate is produced as an intermediate, which subsequently dehydrates to form anhydride. In this study, the thermal dehydration of calcium sulfate hemihydrate under different self-generated p(H2O) conditions was investigated to gain further insight into the reactions in the calcium sulfate–water vapor system. The thermal dehydration of the hemihydrate under two sets of sampling conditions, namely, in open and lidded (semi-closed) pans, was systematically investigated via thermogravimetry (TG) in different heating program modes. The experimentally resolved TG curves were analyzed using the formal kinetic calculation methods based on isoconversional and isothermal kinetic relationships. Under both the sampling conditions, the thermal dehydration reaction was significantly influenced by self-generated p(H2O), which regulated the reaction proceeding from the top surface of the sample bed to the bottom. Under higher self-generated p(H2O) conditions in a lidded pan, the thermal dehydration under different heating program modes exhibited an invariant kinetic behavior characterized by a single set of kinetic parameters, whereas in an open pan the kinetic behavior varied between the reactions under isothermal and other heating modes. Based on the results of the formal kinetic analysis, an advanced kinetic modeling based on a physico-geometrical consecutive reaction model was examined to describe in detail the specific kinetic features of the reaction under self-generated p(H2O) conditions.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...