Reaction of nitric oxide molecules on transition-metal-doped silver cluster cations: size- and dopant-dependent reaction pathways†
Abstract
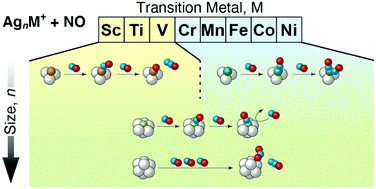
We report size- and dopant-dependent reaction pathways as well as reactivity of gas-phase free AgnM+ (M = Sc–Ni) clusters interacting with NO. The reactivity of AgnM+, except for M = Cr and Mn, exhibits a minimum at a specific size, where the cluster cation possesses 18 or 20 valence electrons consisting of Ag 5s and dopant's 3d and 4s. The product ions range from NO adducts, AgnM(NO)m+, and oxygen adducts, AgnMOm+, to NO2 adducts, AgnM(NO2)m+. At small sizes, AgnMOm+ are the major products for M = Sc–V, whereas AgnM(NO)m+ dominate the products for M = Cr–Ni in striking contrast. In both cases, these reaction products are reminiscent of those from an atomic transition metal. However, the reaction pathways are different at least for M = Sc and Ti; kinetics measurements reveal that the present oxygen adducts are formed via NO adducts, while, for example, Ti+ is known to produce TiO+ directly by reaction with a single NO molecule. At larger sizes, on the other hand, AgnM(NO2)m+ are dominantly produced regardless of the dopant element because the dopant atom is encapsulated by the Ag host; the NO2 formation on the cluster is similar to that reported for undoped Agn+.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...