Atmospheric oxidation of fluoroalcohols initiated by ˙OH radicals in the presence of water and mineral dusts: mechanism, kinetics, and risk assessment†
Abstract
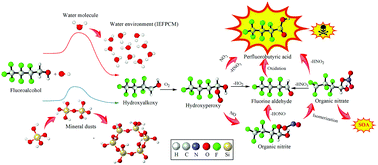
The transport and formation of fluorinated compounds are greatly significant due to their possible environmental risks. In this work, the ˙OH-mediated degradation of CF3CF2CF2CH2OH and CF3CHFCF2CH2OH in the presence of O2/NO/NO2 was studied by using density functional theory and the direct kinetic method. The formation mechanisms of perfluorocarboxylic/hydroperfluorocarboxylic acids (PFCAs/H-PFCAs), which were produced from the reactions of α-hydroxyperoxy radicals with NO/NO2 and the ensuing oxidation of α-hydroxyalkoxy radicals, were clarified and discussed. The roles of water and silica particles in the rate constants and ˙OH reaction mechanism with fluoroalcohols were investigated theoretically. The results showed that water and silica particles do not alter the reaction mechanism but obviously change the kinetic properties. Water could retard fluoroalcohol degradation by decreasing the rate constants by 3–5 orders of magnitude. However, the heterogeneous ˙OH-rate coefficients on the silica particle surfaces, including H4SiO4, H6Si2O7, and H12Si6O18, are larger than that of the naked reaction by 1.20–24.50 times. This finding suggested that these heterogeneous reactions may be responsible for the atmospheric loss of fluoroalcohols and the burden of PFCAs. In addition, fluoroalcohols could be exothermically trapped by H12Si6O18, H6Si2O7, and H4SiO4, in which the chemisorption on H12Si6O18 is stronger than that on H6Si2O7 or H4SiO4. The global warming potentials and radiative forcing of CF3CF2CF2CH2OH/CF3CHFCF2CH2OH were calculated to assess their contributions to the greenhouse effect. The toxicities of individual species were also estimated via the ECOSAR program and experimental measurements. This work enhances the understanding of the environmental formation of PFCAs and the transformation of fluoroalcohols.


 Please wait while we load your content...
Please wait while we load your content...