Strong bases behave as weak bases in nanoscale chemical environments: implication in humidity-swing CO2 air capture†
Abstract
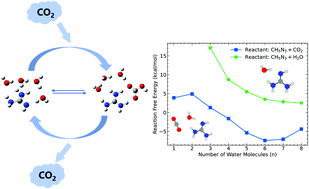
Hydration of ions/molecules in nanometer-sized clusters or nanoscopic pores is ubiquitous and plays a key role in many chemical and physical systems. In this work, guanidine–H2O reactions with n = 1–8 water molecules were systematically studied by ab initio methods. The result suggests that the reduced availability of water molecules greatly inhibits the strong base guanidine from producing OH−. That is, guanidine exhibits the behavior of a weak bases in low-humidity nanoscale environments. Intriguingly, this effect is not limited to guanidine but could be applied to other strong bases. Furthermore, we demonstrate that the direction of guanidine–CO2 reactions can be controlled by changing the number of water molecules present, which in turn responds to the humidity change in air. These findings not only shed some light on unconventional chemical reactions of strong bases in atmospheric clusters and on solid porous surfaces, but also provide insights into the development of guanidine-based CO2 air-capture sorbents.


 Please wait while we load your content...
Please wait while we load your content...