Unique and generic structural features of cholinium amino acid-based biocompatible ionic liquids†
Abstract
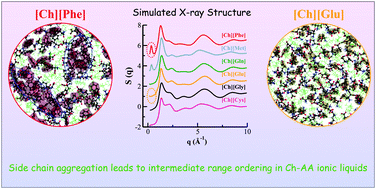
Cholinium amino acid-based (Ch-AA) biocompatible ionic liquids (bio-ILs) are synthesized from renewable components and are efficiently used for biomass processing. However, their microscopic structural features that lead to their application as biomass solvents remain undetermined. Herein, we use atomistic simulations to investigate the structures of six different Ch-AA bio-ILs up to the nanometer length scale and demonstrate that, depending on the anion side chain structure, the respective IL exhibits structural ordering at different length scales. All the six Ch-AA bio-ILs investigated here show a generic feature of having a strong hydrogen bonding network between the hydroxyl group of the cholinium cation and the carboxyl group of the amino acid anions. We illustrate that each of these bio-ILs also displays a unique feature. Distinctive intermediate range structural ordering leads to heterogeneity in methioninate- and phenylalaninate-based ILs caused by the anion side chain segregation. Intermediate range ordering is not observed in glutaminate- and glutamate-based ILs because significant anion side chain and backbone interactions hinder the formation of side chain clusters. Interestingly, for the cysteinate-based IL, the side chains do not interact with the backbones and the intermediate range ordering is not observed because of a shorter anionic side chain.


 Please wait while we load your content...
Please wait while we load your content...