The many-body expansion for aqueous systems revisited: III. Hofmeister ion–water interactions†
Abstract
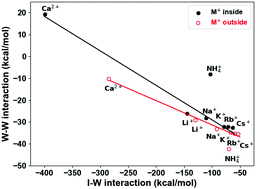
We report a Many Body Energy (MBE) analysis of aqueous ionic clusters containing anions and cations at the two opposite ends of the Hofmeister series, viz. the kosmotropes Ca2+ and SO42− and the chaotropes NH4+ and ClO4−, with 9 water molecules to quantify how these ions alter the interaction between the water molecules in their immediate surroundings. We specifically aim at quantifying how various ions (depending on their position in the Hofmeister series) affect the interaction between the surrounding water molecules and probe whether there is a qualitatively different behavior between kosmotropic vs. chaotropic ions. The current results when compared to the ones reported earlier for water clusters [J. P. Heindel and S. S. Xantheas, J. Chem. Theor. Comput., 2020, 16, 6843–6855] as well as for alkali metal and halide ion aqueous clusters of the same size [J. P. Heindel and S. S. Xantheas, J. Chem. Theor. Comput., 2021, 17, 2200–2216], which lie in the middle of the Hofmeister series, offer a complete account of the effect an ion across the Hofmeister series from “kosmotropes” to “chaotropes” has on the interaction between the neighboring water molecules. Through this analysis, noteworthy differences between the MBE of kosmotropes and chaotropes were identified. The MBE of kosmotropes is dominated by ion–water interactions that extend beyond the 4-body term, the rank at which the MBE of pure water converges. The percentage contribution of the 2-B term to the total cluster binding energy is noticeably larger. The disruption of the hydrogen bonded network due to the dominant ion–water interactions results in weak, unfavorable water–water interactions. The MBE for chaotropes, on the other hand, was found to converge more quickly as it more closely resembles that of pure water clusters. Chaotropes exhibit weaker overall binding energies and weaker ion–water interactions in favor of water–water interactions, somewhat recovering the pattern of the 2-4 body terms exemplified by pure water clusters. A remarkable anti-correlation between the 2-B ion–water (I–W) and water–water (W–W) interactions as well as between the 3-B (I–W–W) and (I–W) interactions was found for both kosmotropic and chaotropic ions. This anti-correlation is linear for both monatomic anions and monatomic cations, suggesting the existence of underlying physical mechanisms that were previously unexplored. The consideration of two different structural arrangements (ion inside and outside of a water cluster) suggests that fully solvated (ion inside) chaotropes disrupt the hydrogen bonding network in a similar manner to partially solvated (ion outside) kosmotropes and offers useful insights into the modeling requirements of bulk vs. interfacial ion solvation. It is noteworthy that the 2-B contribution to the total Basis Set Superposition Error (BSSE) correction for both kosmotropic and chaotropic ions follows the universal erf profile vs. intermolecular distance previously reported for pure water, halide ion–water and alkali metal ion–water clusters. When scaled for the corresponding dimer energies and distances, a single profile fits the current results together with all previously reported ones for pure water and halide water clusters. This finding lends further support to schemes for accurately estimating the 2-B BSSE correction in condensed environments.


 Please wait while we load your content...
Please wait while we load your content...
