Intercalation–exfoliation processes during ionic exchange reactions from sodium lepidocrocite-type titanate toward a proton-based trititanate structure†
Abstract
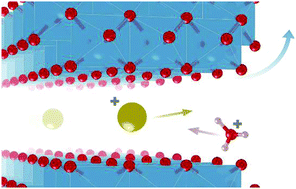
Topochemical reactions involving ionic exchange have been used to assess a large number of metastable compositions, particularly in layered metal oxides. This method encompasses complex reactions that are poorly explored, yet are of prime importance to understand and control the materials’ properties. In this work, we embark on investigating the reactions involved during the ionic exchange between a layered Na-titanate (lepidocrocite-type structure) and an acidic solution (HCl), leading to a protonic (H3O+) titanate (trititanate structure). The reactions involve an ionic exchange provoking a structural change from the lepidocrocite-type to the trititanate structure as shown by real-space refinements of ex situ pair distribution function data. Mobile Na+ ions are exchanged by hydronium ions inducing high proton mobility in the final structure. Moreover, the reaction was followed by ex situ23Na and 1H solid-state MAS NMR which allowed, among other things, confirming that the Na+ ions are in the interlayer space and specifying their local environment. Strikingly, the ionic exchange reaction induces progressive exfoliation of the Na-titanate particles leading to 2–5 nm thin elongated crystallites. To further understand the different steps associated with the ionic exchange, the evolution of the electrolytic conductivity, using conductimetric titration, has been monitored upon HCl addition, enabling characterization of the intercalation(H+)/de-intercalation(Na+) reactions and assessing kinetic parameters. Accordingly, it is hypothesized that the exfoliation of the particles is due to the accumulation of charges at the particle level in relation to the rapid intercalation of protons. This work provides novel insights into ionic exchange reactions involved in layered oxide compounds.


 Please wait while we load your content...
Please wait while we load your content...
