The primary photolysis dynamics of oxalate in aqueous solution: decarboxylation†
Abstract
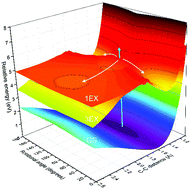
We study the primary reaction dynamics of aqueous oxalate following photo-excitation of the nO → πCO* transition at λ = 200 nm. After the excitation, some of the oxalate molecules return to the electronic ground state on two very different time scales: a fast component of τ = 1.1 ± 0.5 ps comprising 40% of the excited molecules and a much slower component of τ = 0.28 ± 0.05 ns accounting for 15% of the excited molecules. The remaining 45% of the excited molecules do not return to the ground state during the first 500 ps, because they either detach an electron, dissociate or stay excited for hundreds of picoseconds. Dissociation and electron detachment of oxalate predominantly produces CO2 molecules with only minor yields of CO2˙− radical anions. The CO2 formation is accompanied by the ejection of electrons.


 Please wait while we load your content...
Please wait while we load your content...