Catalytic effect of water and formic acid on the reaction of carbonyl sulfide with dimethyl amine under tropospheric conditions†
Abstract
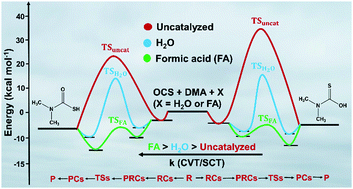
CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ calculations were performed on the addition of amines [i.e. ammonia (NH3), methyl amine (MA), and dimethyl amine (DMA)] to carbonyl sulfide (OCS), followed by transfer of the amine H-atom to either the S-atom or O-atom of OCS, assisted by a single water (H2O) or a formic acid (FA) molecule, leading to the formation of the corresponding carbamothioic S- or O acids. For the OCS + NH3 and OCS + MA reactions with or without the H2O or FA, very high barriers were observed, making these reactions unfeasible. Interestingly, the barrier heights for the OCS + DMA reaction, involving H-atom transfer to either the S-atom or O-atom of OCS and assisted by a FA, were found to be −4.2 kcal mol−1 and −3.9 kcal mol−1, respectively, relative to those of the separated reactants. The barrier height values suggest that FA lowers the reaction barriers by ∼28.4 kcal mol−1 and ∼35.9 kcal mol−1 compared to the OCS + DMA reaction without the catalyst. Rate coefficient calculations were performed on the OCS + DMA reaction both without a catalyst, and assisted by a H2O and a FA molecule using canonical variational transition state theory and small curvature tunneling at the temperatures between 200 and 300 K. The rate data show that the OCS + DMA + FA reaction proceeds through H-atom transfer to the S-atom of OCS, which was found to be ∼103–1011 and 103–1010 times faster than the OCS + DMA and OCS + DMA + H2O reactions, respectively, in the studied temperature range. For the same temperature range, the rate of the OCS + DMA + FA reaction was found to be ∼108–1016 and 103–1012 times faster than the OCS + DMA and OCS + DMA + H2O reactions in which H-atom transfer to the O-atom of OCS occurred. This suggests that the OCS + DMA reaction that is assisted by FA is more efficient than the H2O assisted reaction. In addition, the rate of the OCS + DMA + FA reaction was found to be ∼1010 times slower than the OCS + ˙OH reaction at 298 K. This clarifies that the OCS + DMA + FA reaction may be feasible for the atmospheric removal of OCS under night-time forest fire conditions when the OCS and DMA concentrations are high and the ˙OH concentration is low.


 Please wait while we load your content...
Please wait while we load your content...
