Gas-phase pyrolysis of trans 3-pentenenitrile: competition between direct and isomerization-mediated dissociation†
Abstract
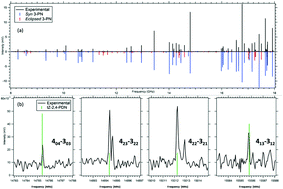
The flash pyrolysis of trans 3-pentenenitrile (3-PN, CH3–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CN) was studied by combining the results of VUV photoionization mass spectra with broadband microwave spectra recorded as a function of the temperature of the pyrolysis tube. The two separated functional groups (vinyl and nitrile) open up isomerization as an initial step in competition with unimolecular dissociation. Primary products were detected by keeping the 3-PN concentration low and limiting reaction times to the traversal time of the gas in the pyrolysis tube (∼100 μs). The reaction is quenched and products are cooled by expansion into vacuum before interrogation over the 8–18 GHz region using chirped-pulse broadband methods. 118 nm VUV photoionization of the same reaction mixture provides a means of detecting all products with ionization potentials below 10.5 eV with minimal fragmentation. These results are combined with a detailed computational investigation of the C5H7N and related potential energy surfaces, leading to a consistent picture of the unimolecular decomposition of 3-PN. Loss of two H-atoms to form a 79 amu product is proven from its microwave transitions to contain trans-Z-2,4-pentadienenitrile, while no pyridine is observed. Methyl loss, HCN loss, and breaking the central C(2)–C(3) bond all occur following isomerization of the position of the double bond, thereby opening up low-energy pathways to these decomposition channels.
CH–CH2–CN) was studied by combining the results of VUV photoionization mass spectra with broadband microwave spectra recorded as a function of the temperature of the pyrolysis tube. The two separated functional groups (vinyl and nitrile) open up isomerization as an initial step in competition with unimolecular dissociation. Primary products were detected by keeping the 3-PN concentration low and limiting reaction times to the traversal time of the gas in the pyrolysis tube (∼100 μs). The reaction is quenched and products are cooled by expansion into vacuum before interrogation over the 8–18 GHz region using chirped-pulse broadband methods. 118 nm VUV photoionization of the same reaction mixture provides a means of detecting all products with ionization potentials below 10.5 eV with minimal fragmentation. These results are combined with a detailed computational investigation of the C5H7N and related potential energy surfaces, leading to a consistent picture of the unimolecular decomposition of 3-PN. Loss of two H-atoms to form a 79 amu product is proven from its microwave transitions to contain trans-Z-2,4-pentadienenitrile, while no pyridine is observed. Methyl loss, HCN loss, and breaking the central C(2)–C(3) bond all occur following isomerization of the position of the double bond, thereby opening up low-energy pathways to these decomposition channels.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
