A test of ab initio-generated, radial intermolecular potential energy functions for five axially-symmetric, hydrogen-bonded complexes B⋯HF, where B = N2, CO, PH3, HCN and NH3
Abstract
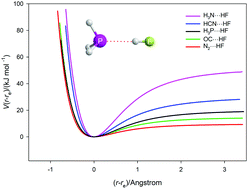
The radial potential energy functions for five axially symmetric, hydrogen-bonded complexes B⋯HF (B = N2, CO, PH3, HCN and NH3) have been calculated ab initio at the explicitly correlated level of theory CCSD(T)(F12c)/cc-pVTZ-F12 as a function of the hydrogen-bond distance r(Z⋯H), where Z is the hydrogen-bond acceptor atom of B. The remaining geometric parameters were optimised at each point in the calculation. The functions so generated were then used to estimate the spectroscopic constants ωσ, ωσxσ,ασ, and the vσ = 1 ← 0 transition wavenumber associated with the intermolecular stretching mode νσ of each B⋯HF by using two equivalent approaches. Both involved the assumption that the vibrational modes of the B and HF molecules were sufficiently stiff relative to the intermolecular stretching mode that B⋯HF could be treated in a pseudo-diatomic approximation. One approach used derivatives of the potential evaluated at the distance r = re while the other used the potential constants obtained by non-linear regression fits of three analytical functions (Morse, Rydberg and Hulburt–Hirschfelder) to the ab initio calculated points. The two approaches would give exactly the same results if the functions were a perfect fit. The H–H function gave the best fit. The determined spectroscopic constants were found to be in reasonable agreement with the limited number available by experiment. The efficacy of the approach was tested for the diatomic molecule H35Cl by taking advantage of both an accurate RKR-type potential and an accurate set of spectroscopic constants. It was also established that the relationship De = kσ/(2a2) between two measures (De and kσ, both calculated ab initio) of the strength of the hydrogen bond in the B⋯HF complexes (and required if the H–H function were an accurate representation of the B⋯HF potential functions) holds to an excellent level of approximation, and supports the conclusion that this function is appropriate to represent the hydrogen bond in the complexes investigated.


 Please wait while we load your content...
Please wait while we load your content...